Challenge: Understanding extracellular vesicle structure
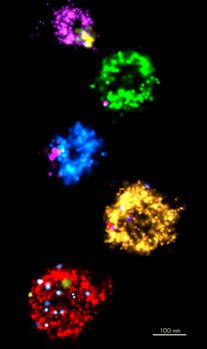 Extracellular vesicles (EVs) play key roles in cell-to-cell communication. EVs can cross biological barriers (such as the blood-brain barrier) and be internalized within the cell with a high degree of specificity. For these reasons, EVs are of significant interest as therapeutic age
Extracellular vesicles (EVs) play key roles in cell-to-cell communication. EVs can cross biological barriers (such as the blood-brain barrier) and be internalized within the cell with a high degree of specificity. For these reasons, EVs are of significant interest as therapeutic age
nts, drug delivery vehicles and diagnostic biomarkers.
EVs typically have a hydrodynamic diameter of 30-200 nm; for the small extracellular vesicles subgroup (historically referred to as exosomes), this value is thought to be 30-120 nm, which is smaller than the theoretically achievable resolution of a conventional light microscope. The fate and interactions of extracellular vesicles inside cells are, therefore, difficult to study, which is limiting researchers in the field.
Recently developed super-resolution techniques, including PALM and dSTORM, overcome the diffraction limit of light and allow for EVs and their contents to be investigated at molecular resolution.
Solution with the Nanoimager: Imaging extracellular vesicles using dSTORM microscopy
The Nanoimager is the world’s first desktop-compatible microscope able to easily visualize EVs with 100x magnification and a resolution reaching 20 nm. Importantly, it uses ultra-high single-molecule sensitivity to track single vesicles with extremely strong signal in fluorescence mode, for sizing and counting using tracking-based methods.
Super-resolution microscopy opens a wide range of possibilities to better understand the mechanisms of extracellular vesicles’ biogenesis and behavior in solution and in cells. Using dSTORM we can now gain detailed knowledge of how and where individual EVs are arranged, localized and clustered.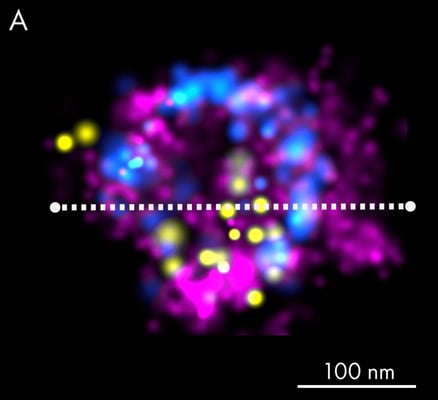
Case Study: Visualizing EVs with exceptional resolution
 Super-resolution imaging can be used to study fine morphological details and precise localization of EV-associated proteins. The presented figure provides an excellent example of the application of dSTORM to investigate extracellular vesicles. Panel A shows an EV that was isolated from human keratinocyte culture media and stained using fluorescently labeled lectins, which specifically bind terminal carbohydrate moieties on the vesicle membrane surface (magenta).
Super-resolution imaging can be used to study fine morphological details and precise localization of EV-associated proteins. The presented figure provides an excellent example of the application of dSTORM to investigate extracellular vesicles. Panel A shows an EV that was isolated from human keratinocyte culture media and stained using fluorescently labeled lectins, which specifically bind terminal carbohydrate moieties on the vesicle membrane surface (magenta).
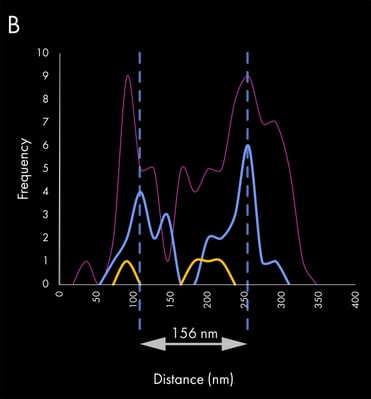
Subsequently, EVs were immunostained with a combination of antibodies against the most commonly known tetraspanins, CD63 (blue) and CD81 (yellow). The estimated size of the example EV is approximately 156 nm, as judged by the CD63 distribution (blue peaks), since lectin staining accounts for an additional 20-40 nm on each side of the particle. Panel B shows the corresponding exosome linescan with the different biomarker frequencies.
Read more about tracking extracellular vesicles or read the full story at the Extracellular Vesicle Hub.
Share this article: